amorphous glass structure
The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica (SiO2) plus Na2O, CaO, and several minor additives. Often, the term glass is used in a restricted sense to refer to this specific use.

Amorphous structure of a
In science, however, the term glass is usually defined in a much wider sense, including every solid that possesses a non-crystalline (i.e., amorphous) structure and that exhibits a glass transition when heated towards the liquid state. In this wider sense, glasses can be made of quite different classes of materials: metallic alloys, ionic melts, aqueous solutions, molecular liquids, and polymers. For many applications (bottles, eyewear) polymer glasses (acrylic glass, polycarbonate, polyethylene terephthalate) are a lighter alternative to traditional silica glasses.

and an amorphous glass:
Glass, as a substance, plays an essential role in science and industry. Its chemical, physical, and in particular optical properties make it suitable for applications such as flat glass, container glass, optics and optoelectronics material, laboratory equipment, thermal insulator (glass wool), reinforcement materials (glass-reinforced plastic, glass fiber reinforced concrete), and glass art (art glass, studio glass).
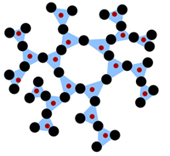
amorphous structure, glass
Ceramics 5a

The amorphous structure of

Amorphous glass structure.

Solid State Structure

Main article: Structure of

glass structure

the amorphous case, i.e.,

Amorphous structure of a
In science, however, the term glass is usually defined in a much wider sense, including every solid that possesses a non-crystalline (i.e., amorphous) structure and that exhibits a glass transition when heated towards the liquid state. In this wider sense, glasses can be made of quite different classes of materials: metallic alloys, ionic melts, aqueous solutions, molecular liquids, and polymers. For many applications (bottles, eyewear) polymer glasses (acrylic glass, polycarbonate, polyethylene terephthalate) are a lighter alternative to traditional silica glasses.

and an amorphous glass:
Glass, as a substance, plays an essential role in science and industry. Its chemical, physical, and in particular optical properties make it suitable for applications such as flat glass, container glass, optics and optoelectronics material, laboratory equipment, thermal insulator (glass wool), reinforcement materials (glass-reinforced plastic, glass fiber reinforced concrete), and glass art (art glass, studio glass).

amorphous structure, glass
Ceramics 5a

The amorphous structure of

Amorphous glass structure.

Solid State Structure

Main article: Structure of

glass structure

the amorphous case, i.e.,

0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home